The National Rosacea Society (NRS) and American Acne and Rosacea Society (AARS) are celebrating Rosacea Awareness Month this April by providing public education to reach the many millions of rosacea sufferers who may not realize they have a medical condition that can be treated, emphasizing the warning signs and urging those who suspect they may have rosacea to see a dermatologist.
Related: Rosacea Awareness Month Highlights the Importance of Rosacea-safe Skin Care
During Rosacea Awareness Month, AARS continues to educate its members and their patients about the signs and symptoms of this common inflammatory skin disease that most often affects the face. AARS members are sharing #RosaceaFacts and highlighting the availability of effective treatments for the condition. As part of its efforts, AARS has published a social media toolkit for use by its professional members. The kit includes social posts, cards and banners for use on various social media platforms and inspiration for informative social posts.
“It's very important that patients who have rosacea or think they have rosacea go to board-certified dermatologists or licensed healthcare professionals within a dermatology practice, such as physician assistants and nurse practitioners who are educated in dermatology,” says AARS president James Q. Del Rosso, DO. “There is no reason patients should not receive treatment, especially as we continue to see effective, tolerable new therapies come to market.”
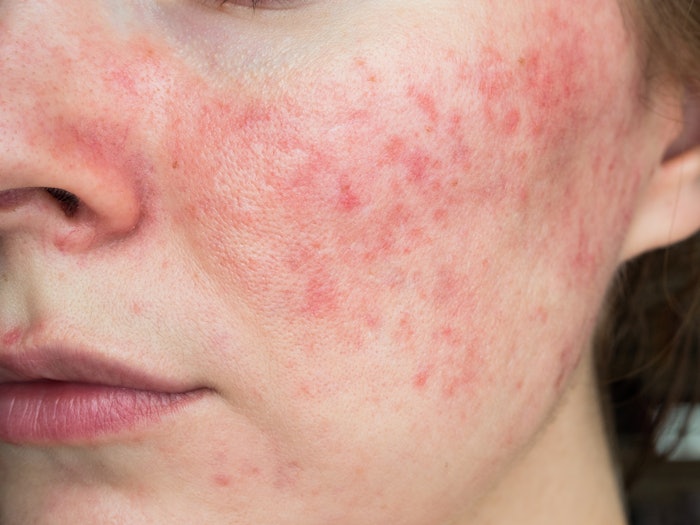
Per NRS, the most common individual sign of rosacea is persistent redness on the central part of the face – the cheeks, nose, forehead and chin. The redness may be accompanied by itching, burning or stinging, and sufferers may also experience bumps and pimples or develop tiny visible blood vessels on their skin. Prior to diagnosis, many rosacea sufferers may turn to skin care products and cosmetics as a way to treat or cover up these signs and symptoms, mistaking them for sunburn or acne.
“The general consensus among dermatologists today is that, in addition to medical care, effective skin care recommendations are a crucial part of successful rosacea therapy,” said Hilary Baldwin, M.D., associate professor of dermatology at Rutgers Robert Wood Johnson Medical School and a member of the NRS medical advisory board. “The use of quality OTC products can not only help improve the signs and symptoms in and of themselves but are often used as adjuncts before and during prescription therapy and as part of long-term care.”
In NRS surveys, 92% of rosacea patients reported burning, stinging and itching, 82% reported that certain skin care products and cosmetics aggravated their condition, 70% said there were specific ingredients that irritated their skin and 84% were interested in more guidance on skin care. The most common irritants were astringent alcohol (63%), perfumes or fragrances (57%), witch hazel (31%) and menthol (30%). Around a quarter of respondents were affected by peppermint or eucalyptus oil, dyes and pigments, sulfates, and parabens or other preservatives.
“While the use of skin care and cosmetic products can help minimize the appearance and effects of rosacea, poorly selected products may make it worse.” Dr. Baldwin said. “Fortunately, there are now many products that are formulated to be suitable for sensitive, rosacea-prone skin, without ingredients that may cause flare-ups. The primary challenge today is when rosacea sufferers face the bewildering array of options in the pharmacy skin care aisle.”
NRS recently launched a Seal of Acceptance program to identify skin care and cosmetic products that are beneficial to patients with rosacea. The new program was developed under the guidance of Zoe Draelos, M.D., a clinical and research dermatologist, former vice president of the American Academy of Dermatology and president of Dermatology Consulting Services, PLLC. To be considered for the Seal, skin care and cosmetic products must be free of ingredients that cause skin barrier dysfunction, flushing (vasomotor instability) or unwanted neurosensory stimulation such as burning or itching.
Each product earning the NRS Seal of Acceptance must undergo clinical testing to demonstrate safety and low risk for irritation and sensitization in people with rosacea. Applications, including study data, are anonymously reviewed by an independent panel of dermatologists to determine whether they are approved as acceptable. The NRS Seal of Acceptance is displayed on the packaging of approved products.











