FDA Clearance for DermaSensor's Skin Cancer Evaluation Device
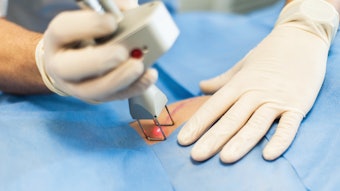
DermaSensor Inc. has announced FDA clearance for its real-time, non-invasive skin cancer evaluation system. It is the first AI-powered medical device that detects all three common skin cancers (melanoma, basal cell carcinoma and squamous cell carcinoma)
DermaSensor’s AI-powered spectroscopy technology can non-invasively evaluate cellular and subcellular characteristics of a lesion in question for skin cancer. The wireless, handheld device provides an immediate result using an FDA-cleared algorithm.
Primary care physicians' options for evaluating suspicious moles have traditionally been the naked eye or magnified visual examination of lesions, which can be dependent on clinical training and subjective judgment.
Related: Nonablative Fractional Laser Treatments May Help Prevent Non-Melanoma Skin Cancer
The FDA's pivotal study led by the Mayo Clinic to validate device performance showed that the device had a sensitivity of 96% across all 224 skin cancers. A negative result had a 97% chance of being benign for all skin cancers. In a companion clinical utility study with 108 physicians, the DermaSensor device was found to decrease the number of missed skin cancers by half (from 18% to only 9%), increasing the physicians’ accuracy and confidence in assessing cancerous lesions. DermaSensor has conducted 13 clinical studies in the last decade, six of which provided the principal support for FDA clearance.
“We are entering the golden age of predictive and generative artificial intelligence in healthcare, and these capabilities are being paired with novel types of technology, like spectroscopy and genetic sequencing, to optimize disease detection and care,” said Cody Simmons, co-founder and chief executive officer of DermaSensor. “Equipping PCPs, the most abundant clinicians in the country, to better evaluate the most common cancer in the country has been a major, long-standing unmet need in medicine. While dozens of companies have attempted to address this problem in recent decades, we are honored to be the first device cleared by the FDA that provides PCPs with an automated tool for evaluation of suspicious lesions.”
“Achieving this medical milestone is a testament to the 12 years and tens of millions of dollars our company has invested in research and development to bring this powerful technology to market,” said Maurice Ferre, M.D., co-founder and chairman of DermaSensor. “We are incredibly grateful to the FDA for their collaboration and dedication to this area starting with our first FDA pre-submission meeting in 2016. Having begun patient enrollment in our FDA pivotal study in mid-2020, we are now ecstatic to have clearance of our FDA-breakthrough designated de Novo submission.”
Moderna and Merck Developing mRNA Vaccine for Melanoma
Moderna has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. The presented mid-term data from the 3-year follow-up is a somewhat promising development in the treatment of melanoma.
The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection. Relapse Risk Halved Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone, per the study.
The combination of mRNA-4157 (V940) with pembrolizumab also demonstrated a meaningful improvement in distant metastasis-free survival compared with pembrolizumab alone, reducing the risk of developing distant metastasis or death by 62%, per the study.
“The KEYNOTE-942/mRNA-4157- P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president.
The combined treatment did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%).
Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the FDA and European Medicines Agency awarded a breakthrough therapy designation and recognition under the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].”
An mRNA vaccine for melanoma could be available in 2025, according to Stéphane Bancel, Moderna’s director general. Other cancer vaccines in clinical trial phases include the partnership between BioNTech and Roche targeting pancreatic cancer in nature, Ose Immunotherapeutics' vaccine for advanced lung cancer and Transgene's viral vector vaccines against ENT and papillomavirus-linked cancers.