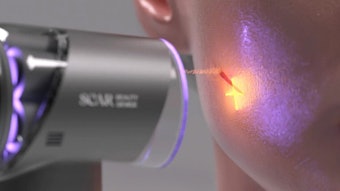
Syneron Medical has received 510(k) clearance from the United States Food and Drug Administration (www.fda.gov) to market its me Home-Use Hair Removal System to consumers. The me hair removal system is the first and only FDA cleared consumer hair removal technology that is approved for all skin tones. It is also the first home-use product featuring Syneron's proprietary elos technology—which combines intense pulse light (IPL) and radiofrequency (RF) energies—to receive FDA clearance. The device is currently CE Marked, approved by Health Canada, and has been commercially available in several European markets since 2011.
“The me system is the only consumer hair removal technology that is approved for all skin tones, increasing the addressable consumer market and underscoring the technological advantages of our proprietary elos technology for home-use aesthetic products,” said Fabian Tenenbaum, CEO of Syneron Beauty. “The me system has been well received in Europe and we are confident that consumers in the U.S. will be similarly attracted to its strong efficacy and unmatched comfort and safety profile.”
Jerome M. Garden, MD, Department of Dermatology, Northwestern University, added, “The Company conducted a 100-patient clinical study, covering all skin colors, at four leading dermatologist sites in the U.S., using the me hair removal instrument. The data from the study supports efficacy across all skin tones and confirmed a significant safety profile.”
Syneron Home Use Hair Removal Device Approved
Oct 18th, 2012