Sebacia announced that it has completed a $20 million Series D financing to complete a U.S. pivotal trial of its proprietary microparticle treatment for moderate to severe acne.
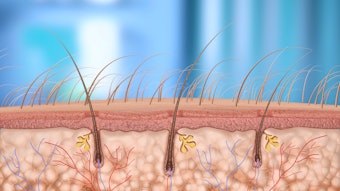
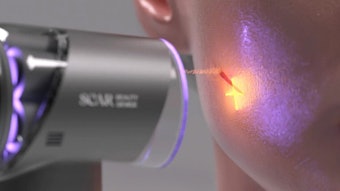
The company's gold and silica microparticles are specially designed to be activated by the light from commonly used hair removal lasers and are placed in a suspension designed to penetrate the sebaceous follicles. When exposed to a laser pulse, they create a focused photothermal effect in the sebaceous gland and follicle to reduce the activity level of the gland and the inflammatory lesions that cause acne. The treatment is intended to be a simple, physician-guided, in-office procedure that could provide an alternative to existing therapies, such as oral antibiotics and isotretinoin.
With the new funding from Versant Ventures, Domain Associates, Accuitive Medical Ventures, Partners Healthcare Innovation Fund, Salem Partners and other undisclosed investors, Sebacia intends to complete the U.S. pivotal trial with expected results reporting by mid-2018, and submission and FDA response expected by the end of 2018. Additionally, the company plans to expand its worldwide patent estate, which includes the seminal selective photothermalysis methods developed by Dr. R. Rox Anderson at Massachusetts General Hospital.
“At this pivotal time, we are grateful for the continued support of our top-tier venture capital syndicate and energized by the addition of Salem Partners and other new investors,” said Anthony V. Lando, CEO of Sebacia. “From intellectual property to extensive clinical experience and a defined clinical path to approval, we believe we have all the pieces to bring Sebacia's breakthrough treatment for acne to market in Europe and the United States.”