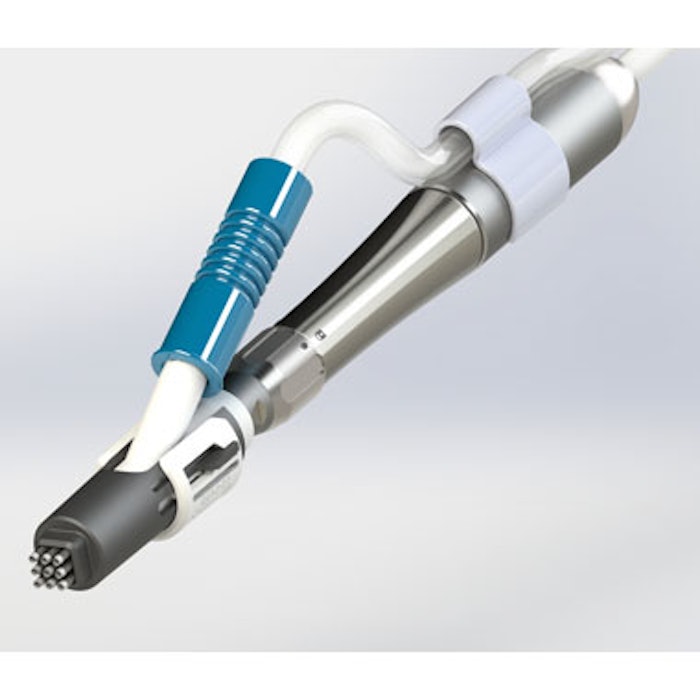
Recros Medica’s de novo regulatory submission for the Nuvellus Focal Contouring System has been accepted for review by the U.S. Food and Drug Administration (FDA). The device utilizes the company’s proprietary Rotational Fractional Resection (RFR) technology, which creates fractional wounding without heat or energy and may provide medical aesthetic physicians a minimally invasive single treatment option to improve submental contouring.
The system will be comprised of an integrated console, reusable surgical handpiece and differentiated consumable attachments for specific contouring applications. Based on anticipated regulatory timelines, Recros expects to receive FDA clearance in Q4 2019.
“The entire Recros Medica team is excited about bringing our novel Nuvellus system to aesthetic physicians, and today’s submission represents a critical step in that journey,” said Tom Albright, CEO of Recros Medica. “We are confident that Nuvellus will offer customers significant efficacy and versatility, and look forward to working with the FDA to achieve commercial clearance and introduce Nuvellus for one of the largest unmet needs in aesthetic medicine.”
For more information, visit https://www.recrosmedica.com.











