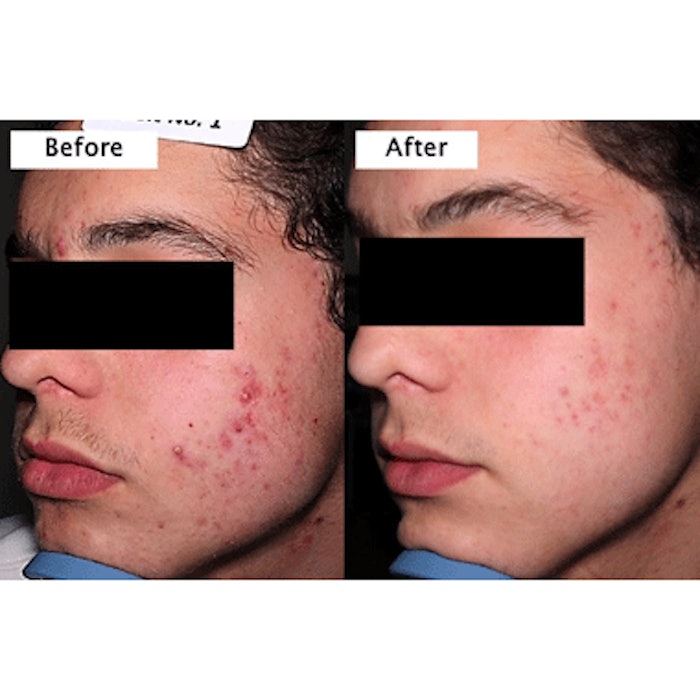
Foamix, a clinical stage specialty pharmaceutical company, has completed Phase II clinical trials of its Minocycline Foam 4%, a first topical minocycline product that can be used for the treatment of acne, rosacea and skin infections. The double blind, dose ranging, placebo controlled study followed 150 patients with moderate to severe acne. Patients were randomized into three equal groups of 50 patients, who received placebo, or one of two Minocycline Foams--1% or 4%.
Once daily treatment with Minocycline Foam 4% exhibited an average 71% reduction in inflammatory lesions after six weeks with a respective reduction of 55% in non-inflammatory lesions. At the end of the 12-week treatment period, the reduction in inflammatory lesions was at 72% with the reduction in non-inflammatory lesions reaching 73%. The effects were dose-dependent, as demonstrated by lower effects seen with 1% foam and placebo.
The Investigator’s Global Assessment (IGA) scores also significantly improved during the trial. At 12 weeks, 53% of the patients had an IGA score of either “Clear” or “Almost clear,” while only 20% of the placebo-treated patients had the same scores.
Among patients in the Minocycline 4% group, 61% stated that the efficacy was very high or high, and 27% rated the efficacy as moderate (median efficacy rating in the placebo group was “moderate”).
Adverse events included mild and transient skin dryness, peeling and/or erythema, evenly distributed among the treatment groups. No drug-related systemic adverse events were noted.
Photo courtesy of Foamix











