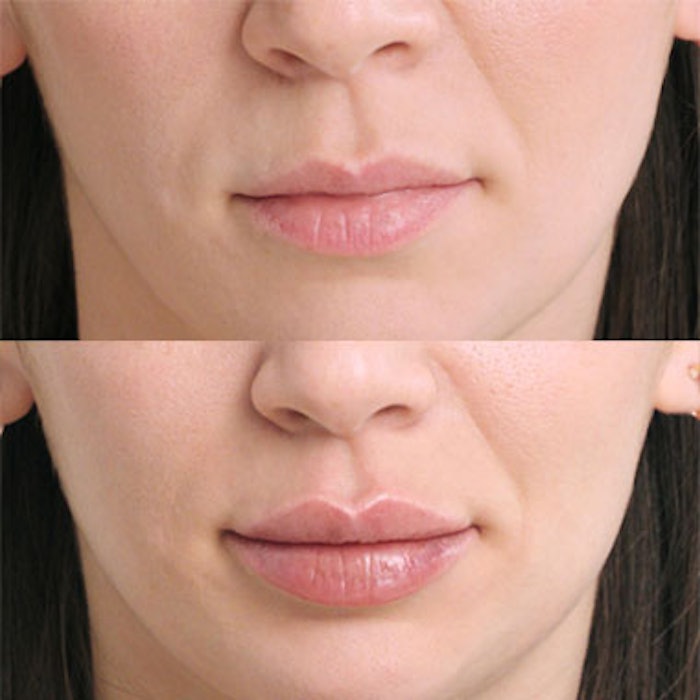
The U.S. Food and Drug Administration (FDA) has approved Galderma’s Restylane Kysse for lip augmentation and the correction of upper perioral rhytids in adults over the age of 21. Restylane Kysse is a hyaluronic acid (HA)-based filler that lasts for up to one year and is indicated for people with lips that have changed due to the aging process or for those seeking natural-looking, fuller lips.
“Restylane Kysse is a new lip filler that offers key attributes both providers and their patients desire in a lip injection—high satisfaction, consistent results and a proven clinical-safety profile,” said Alisa Lask, general manager and vice president of U.S. Aesthetic Business at Galderma. “We are confident that Restylane Kysse will become a leading lip filler in the dynamic U.S. market.”
As with Restylane Refyne and Defyne, Kysse is manufactured using the company’s XpresHAn Technology, which customizes the degree of HA crosslinking in each product, resulting in injectable gels with a range of flexibility and support characteristics for different patient needs. It also contains lidocaine to decrease pain and reduce discomfort associated with injections in the lip area.
In phase 3 clinical trials 78 percent of subjects reported they were still satisfied with their results after one year. In addition, Restylane Kysse was shown to be both safe and well tolerated.
“Restylane Kysse is a game changer with a formulation specifically designed to provide excellent outcomes in the lips,” said Dr. Melanie Palm, board-certified dermatologist and cosmetic surgeon in San Diego, CA and a clinical investigator in the Restylane Kysse phase 3 trial.
To learn more, visit www.RestylaneUSA.com.
Patient before and after Restylane Kysse (.75 mL) injection for lip augmentation and the correction of upper perioral rhytids.











