Due to the growing popularity of skin needling, the U.S. Food and Drug Administration (FDA) has undertaken an examination of several microneedling devices to determine if they conform to government regulations. As advocates of microneedling and distributors of such products, we feel it is important for providers to be aware of FDA guidelines and recent action. Two companies (one with a manual instrument and the other with a motor-driven device) have received warnings or import alerts. Our company (DermaConcepts) and others have been told to cease and desist selling medical needling devices with needles longer than 0.3mm until we obtain proper clearance or approval. We’ve also received warnings that individuals using unapproved devices are exposing themselves to potential legal and licensing problems. DermaConcepts is currently working with the FDA to determine the way forward on devices with needles longer than 0.3mm.
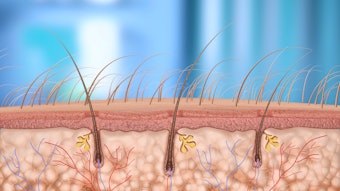
It is important to note that the FDA has not published any regulation specifically on skin needling. It has, however, taken action against companies for selling unapproved medical devices. These actions are based on the FDA’s view that a) needling instruments that break the skin affect the form and function of the body and are, in every sense, medical devices that require clearance as a new medical device as no predicate devices exist; and b) to avoid classification as a medical device, cosmetic needling instruments must have a needle length of 0.3mm or less and make no medical claims in any manner. If a device makes no medical claims, focuses only on improving the appearance of the skin and does not pass the stratum corneum, it does not fall under the jurisdiction of the FDA.
Classifications for Needling Devices
Anyone can get a device or instrument listed (or registered) by completing a simple form and filing it with the FDA. This does not mean the device is approved, nor does it connote that it is legal to be sold or used in the U.S.
The fact that a company lists a device as a Class One medical device—low risk and least regulatory controls—does not mean that the FDA agrees with that designation. In fact, in official correspondence, the FDA rejects the assertion that skin needling devices are Class One simply because a company claims that it is akin to dermabrasion or other similar brushes and tools.
The FDA has instead ruled that skin needling devices (both manual and motorized) with a needle length over 0.3mm or adjustable lengths are Class Two or Class Three devices and, as such, have to go through FDA premarket approval or clearance.
Devices that have a needle length of 0.3mm or more are faced with a challenge, as there are no existing needling device approvals upon which to base a 510(k) approval. Therefore, the manufacturer would need to file an application with the FDA as a new medical device.
In addition to needle length, the FDA classifies devices based on manufacturer claims or marketing materials that state that an instrument or device can alter the form and function of the body. If a device has a needle length of less than 0.3mm, but the manufacturer makes claims that it can alter the form and function of the body, then the device is classified as Class Two and must prove its claims through clinical trials.
For providers, the distinction in needle length is important. Needles that are longer than 0.3mm will most likely draw blood. In a survey of 50 states conducted by DermaConcepts in 2014, 46 states responded that it is illegal for estheticians to use any instrument with needles that can pass through the stratum corneum. Therefore, providers must be sure to understand the characteristics of the device they are purchasing as well as state regulations regarding which devices may be operated by nonmedical personnel.
Carol and Robert Trow are the owners of DermaConcepts.
Photo copyright Getty Images.