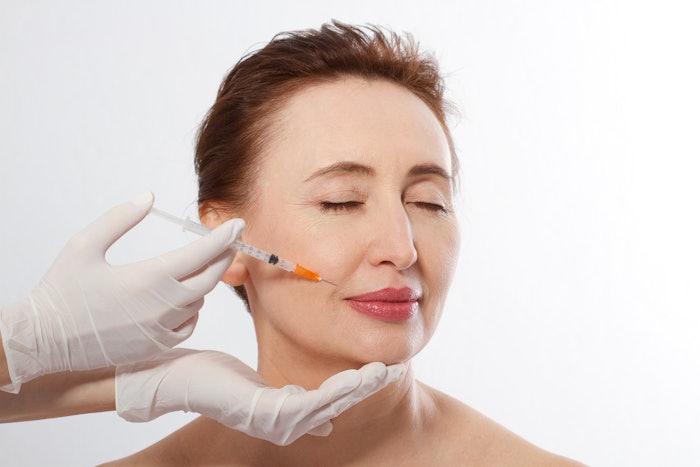
Galderma has announced the European re-launch of Sculptra (injectable poly-L-lactic acid), a collagen stimulator with an updated administration protocol.
Sculptra can now be used immediately following a two-minute reconstitution, where previously healthcare professionals had to wait two hours to administer the product. The updated usage comes from new data from physiochemical studies, and results from a randomized, evaluator-blinded, parallel-group, multi-center study evaluating the safety and effectiveness of two different dilutions of Sculptra. The study found using the product right after reconstitution caused less pain on injection and was well-tolerated.
“We are delighted to bring this expanded Sculptra offering to healthcare professionals in Europe,” said Alexandre Brennan, global business unit head of aesthetics at Galderma. “We continue to build a unique and extensive aesthetics offering at Galderma, and are proud to relaunch Sculptra in Europe as the foundation of our portfolio alongside a range of complementary treatments to deliver impressive and long-lasting results for healthcare professionals and their patients.”