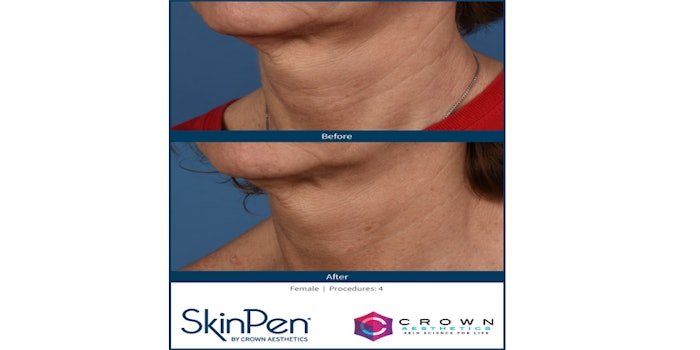
The U.S. Food and Drug Administration (FDA) has cleared Crown Aesthetics' SkinPen Precision microneedling device for the treatment of wrinkles on the neck.
The FDA clearance follows a clinical study, which demonstrated an improvement of dermal lines on the neck and high patient satisfaction at one month and three months post treatment.
The clinical study revealed:
- 94% of patients noticed an improvement in how their wrinkles looked in their treated area one month post procedure
- 88% of patients were satisfied with their SkinPen Precision treatment one month post procedure
- SkinPen Precision device is safe for use with a depth of up to 2.5 mm on the neck











