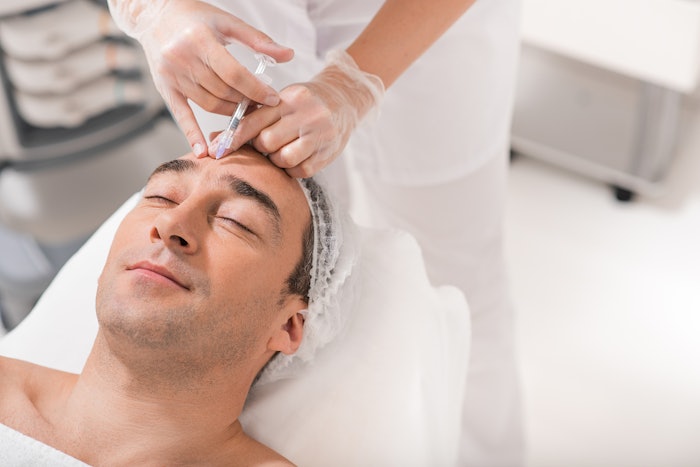
Revance Therapeutics announced that the U.S. Food and Drug Administration (FDA) has accepted its Biologics License Application (BLA) resubmission for the company's DaxibotulinumtoxinA for injection for the treatment of moderate to severe glabellar lines.
The FDA designated the BLA as a Class 2 resubmission, which has a six-month review period and includes a reinspection of the company’s manufacturing facility. Revance was provided a Prescription Drug User Fee Act (PDUFA) goal date of September 8, 2022.











