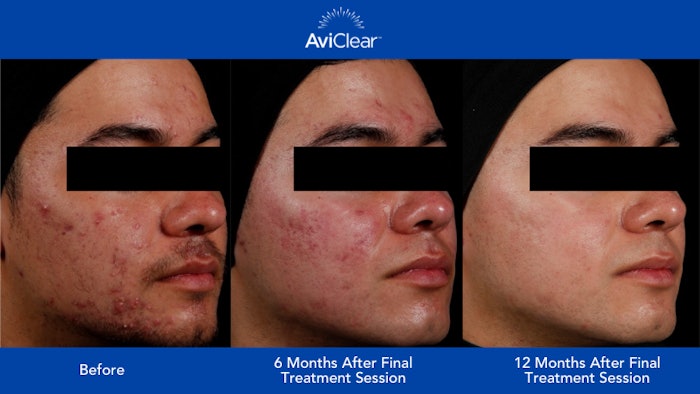
AviClear is positively disrupting the dermatology industry by rivaling traditional methods of acne care with a novel treatment approach and practice-friendly business model. Its unique 1726 nm wavelength treats acne at the source by selectively targeting and suppressing the sebaceous glands without the need for prescriptions, topicals or downtime. Current clinical studies show that after three 30-minute treatment sessions, 90% of patients had a visible improvement in their acne at 6 months.1 New 12-month clinical findings show this improvement increases to 92%,2 confirming the continual improvement of acne clearance and skin quality over time.
Studies also show that three-fourths of patients showed a 2+ IGA score improvement and two-thirds of patients were assessed as clear or almost-clear 12 months after their final treatment session.3
“We are delighted the research supports AviClear’s long-term durability. Many of my patients are thrilled with their results, and with this data, we can visibly display the longstanding effectiveness of the treatment outcomes. This is especially impactful for acne sufferers who are seeking an alternative to isotretinoin; this data proves that AviClear can be that solution for them,” said David J. Goldberg, MD, JD, board-certified dermatologist.
Acne vulgaris is a nearly universal skin disease, with approximately 50 million North American teens and young adults seeking treatment each year.4 Safe for all skin types, AviClear is a game changer for those seeking a chemical-free acne solution without the harmful side effects. This laser treatment is also meeting the demand for the health, wellness and convenience-driven patients, especially the demographic of Gen Z and Millennials seeking long-lasting clinically proven results. To find out how you can become a provider, visit AviClear.com to sign up for product updates and availability.
1,2,3 Data on file. FDA clearance study. Cutera, Inc.
4 https://jamanetwork.com/journals/jamadermatology/fullarticle/479093
Disclaimer:
The above paid-for content was produced by and posted on behalf of the Sponsor. Content provided is generated solely by the Sponsor or its affiliates, and it is the Sponsor’s responsibility for the accuracy, completeness and validity of all information included. MedEsthetics takes steps to ensure that you will not confuse sponsored content with content produced by MedEsthetics and governed by its editorial policy.