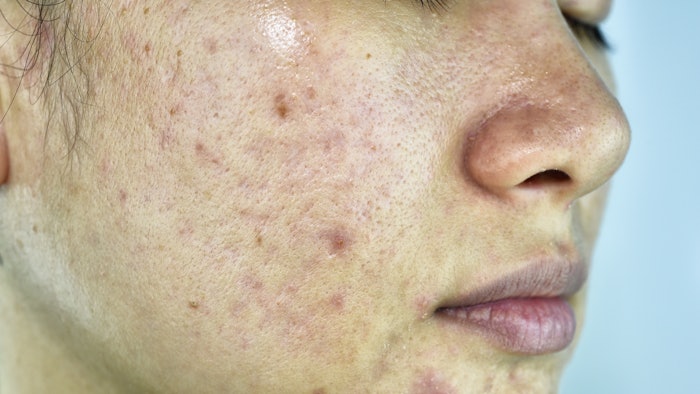
The China National Medical Products Administration (NMPA) has approved the Phase II clinical trial application of ASC40, a fatty acid synthase (FASN) inhibitor, from Ascletis Pharma for the treatment of patients with moderate to severe acne.
Related: Extractions vs. Oral Doxycycline for Acne
FASN is a key enzyme which regulates de novo lipogenesis. Human sebum production requires de novo lipogenesis, which is increased in acne and suppressed by the FASN inhibitor ASC40. Acne is the third indication approved for ASC40 clinical trials in China. The other two indications are non-alcoholic steatohepatitis (NASH) and recurrent glioblastoma (GBM).
The Phase II trial is a randomized, double-blind, placebo-controlled, multicenter clinical trial to be conducted in China to evaluate the safety and efficacy of ASC40 for the treatment of patients with moderate to severe acne vulgaris. About 180 patients are planned to be enrolled and randomly assigned to one of four cohorts (placebo, 25 mg, 50 mg, 75 mg) at the ratio of 1:1:1:1.
Related: Sun Pharma To Distribute Winlevi for Acne in U.S.
"I am very pleased to participate as an investigator in the Phase II clinical trial of ASC40 for the treatment of patients with moderate to severe acne vulgaris," said Professor Ai'e Xu, Director of Hangzhou Institute of Dermatology. "Based on data from previous preclinical and clinical studies, I look forward to positive results from the Phase II clinical trial of ASC40, a first-in-class drug with a novel mechanism of action."











