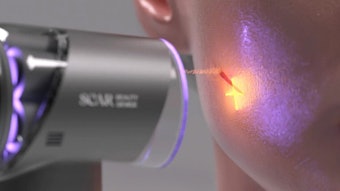
The U.S. Food and Drug Administration (FDA) has approved Sofwave Medical's Sofwave device for three additional indications: lifting the eyebrow, and lifting lax submental tissue and neck tissue for subjects aged 22 and older.
The Sofwave system featuring SUPERB technology was previously cleared by the FDA for a noninvasive dermatological aesthetic treatment to improve facial lines and wrinkles.
Sofwave Medical conducted a multi-site, clinical study that evaluated the safety and effectiveness of the device to lift the eyebrow and lift lax submental and neck tissue. A total of 80 subjects received treatments, and 467 facial areas were treated during the study.
"The FDA clearance represents another major achievement for the Sofwave platform and showcases Sofwave Medical's ongoing focus on innovation and clinical advancements in energy-based technology," said Dr. Shimon Eckhouse, chairman of the board of directors and co-founder of Sofwave Medical. "The SUPERB (Synchronous Ultrasound Parallel Beam) is a unique, industry-leading technology offering practitioners a noninvasive treatment for high demand lifting treatments of face and neck. We will continue to develop innovative products that advance the medical aesthetics industry and meet the needs of our customers and the needs of their patients."