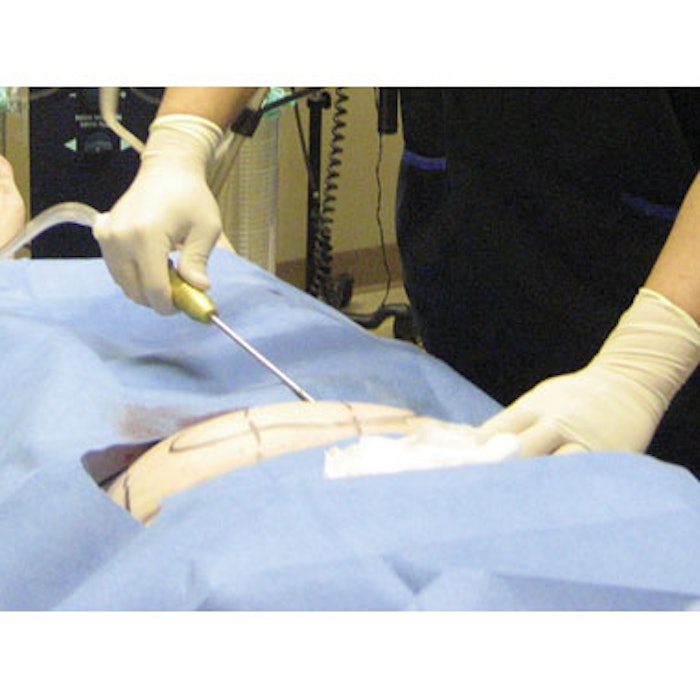
In response to recent reports of myobacterial infections related to liposuction and fat grafting procedures, the International Confederation for Plastic, Reconstructive and Aesthetic Surgery (IPRAS) has released guidelines on the proper processing of multiple-use cannulas. Cannulas must be thoroughly cleaned—inside and out—to remove all tissue, with special attention paid to non-visible areas. Next, all cleaning solutions must be thoroughly removed from the cannula to prevent degradation of the materials. Finally, the cannula must be autoclaved at the “appropriate settings to eliminate bacteria and minimize mycobacterium, prions and biofilms,” says the IPRAS. Since some cleaning agents can degrade plastic and metal materials, all cannulas should be inspected for corrosion and damage. If there are signs of damage, the cannula should not be used. Since the cleaning process is labor-intensive and highly susceptible to human error, the IPRAS recommends that “if suitable reprocessing of multiple-use cannulas is not available, single-use cannulas should be considered.” The guidelines appeared in IPRAS Journal (Issue 3, 2011).
Image courtesy of Jeffrey Lui, MD