FDA Safety Communication
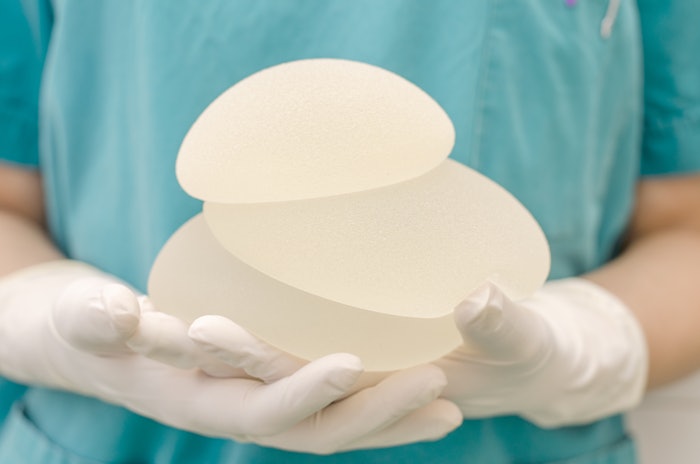
The US Food and Drug Administration (FDA) issued an official safety communication informing providers and patients about the reports of squamous cell carcinoma (SCC) and various lymphomas located in the capsule or scar tissue around breast implants.
There was an extensive review performed, and the FDA believes that the risk of SCC and other lymphomas occurring in the tissue around breast implants is "rare." In this instance, and when safety risks with medical devices are identified, the FDA wanted to provide "clear and understandable information" to the public.
Related: Revance Therapeutics Receives FDA Approval for DAXXIFY
Reports of Lymphoma Scar Tissue
There were some reported cases where patients were diagnosed years after having breast implants and presented with swelling, pain, lumps or skin changes. The new reports of lymphoma in scar tissue differ from Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL), which the FDA identified as a potential risk more than 10 years ago.
For the time being, the FDA does not have enough information to confidently say that breast implants cause these cancers, or if some implants have a higher risk than others. Moving forward, cases of SCC, lymphoma and any cancer found in the scar tissue around breast implants should be reported to the agency.
The FDA plans to complete a comprehensive literature review and continue their partnership with the American Society of Plastic Surgeons. The organizations will work together to identify ways to collect more detailed information on patient cases where cancer in the breast implant capsule has been reported.