Pivotal trials of botulinum toxin type A (BoNTA) injectables often require treatment-naïve subjects. Yet, in real-life clinical practice a large portion of patients seeking these treatments are repeat BoNTA patients. Considering this, a group of dermatologists investigated whether prior BoNTA treatment affects the efficacy, duration of response, placebo response and tolerability of BoNTA for treatment of glabellar lines.
They note that the rationale for prioritizing treatment-naïve subjects for trials is that patients who have previously received BoNTA treatment may artificially lower the placebo efficacy rate, as experienced patients will compare their outcomes with actual treatment (vs. those without prior experience). In addition, experienced subjects may be more aware of potential side effects and, therefore, less concerned or likely to report them to the investigators.
To determine whether these concerns are founded, Joel L. Cohen, MD, et al, reviewed data from the Phase 3 SAKURA clinical development program of DaxibotulinumtoxinA for Injection (DAXI, Revance Therapeutics), which included 2,823 participants receiving a total of 4,444 treatments. The two double-blind, placebo-controlled trials (SAKURA 1 and SAKURA 2) as well as the larger, open-label safety study (SAKURA 3) were unique in that they were not restricted to BoNTA treatment-naïve patients; 40% to 50% of participants in each study had experience with BoNTA treatments. (Previously treated subjects were required to have a washout period of ≥6 months for treatments to the face and ≥3 months for doses >200U anywhere in the body.)
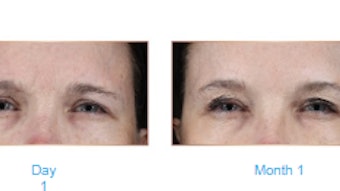
The results of the study, published in Dermatologic Surgery (April 2021) showed that the duration of response between treatment-naïve and treatment-experienced patients was similar in both the DAXI and placebo groups. The incidence of adverse events was also comparable regardless of prior BoNTA treatment status.
The authors concluded that “Assuming an appropriate washout is observed, future BoNTA trials should enroll both treatment-experienced and treatment-naive participants to reflect clinical practice.”