Galderma Labs conducted a clinical trial examining the effectiveness of its Relfydess (RelabotulinumtoxinA), a liquid neuromodulator using Pearl technology in treating glabellar lines, also known as “frown lines,” and lateral canthal lines, or “crow’s feet.” The analysis of the trial was presented at the 2025 International Master Course on Aging Science (IMCAS), according to a Jan. 31 press release.
“While neuromodulators have been used to improve wrinkles for more than 30 years, there has been a historic lack of innovation in this time, meaning patients and healthcare professionals have had to settle for traditional manufacturing and reconstitution processes that can limit performance, ease-of-use and reliability,” says Baldo Scassellati Sforzolini, global head of research and development at Galderma.
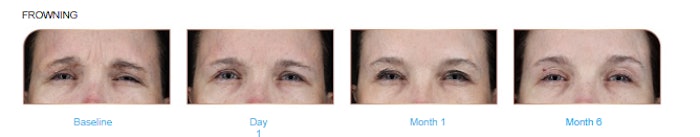
The “phase IIIb Relax” clinical trial was multicenter, randomized, double-blind and placebo-controlled, designed to evaluate the long-lasting efficacy and satisfaction of Relfydess in 132 adults with moderate-to-severe frown lines over a 12-month period, per the release.  The data collected from the clinical trial showed that 39% of patients reported improvements for frown lines and 34% reported improvements for crow’s feet at day one.Courtesy of Galderma Labs
The data collected from the clinical trial showed that 39% of patients reported improvements for frown lines and 34% reported improvements for crow’s feet at day one.Courtesy of Galderma Labs
Within the liquid neuromodulator, Galderma uses precipitation-free extraction and activity-preserving refined liquid (Pearl) technology designed to preserve molecule integrity to deliver a highly active, innovative, complex-free molecule to frown lines.
“This advanced technology, with a unique, multi-step purification process, eliminates precipitation and freeze-drying steps associated with traditional methods, keeping the core molecule in a liquid state throughout,” Sforzolini says. “It is designed to carefully remove complexing proteins and impurities…with the aim of optimizing both activity and purity to produce a highly active formulation…[that] enables simple volumetric dosing, without reconstitution…”
The data collected from the clinical trial showed that 39% of patients reported improvements for frown lines and 34% reported improvements for crow’s feet at day one, Sforzolini says. About 75% of patients maintained improvements for six months in frown lines. After one month post-treatment, 96% achieved none-or-mild frown lines and crow’s feet–sustained for six months in nearly a quarter of patients, he adds.
“First results from our phase IIIb Relax study presented at the IMCAS 2025 annual congress in January reinforce the phase III Ready data, showing Relfydess’ six-month clinical effect and rapid onset as soon as day one,” Sforzolini says.