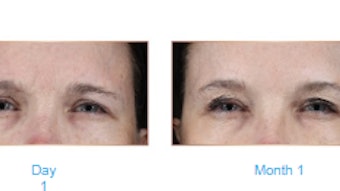
Researchers performed a post hoc analyses of pooled data from the 492 prabotulinumtoxinA-treated patients who participated in the two U.S.-based multicenter, randomized, double-blind, placebo-controlled, single-dose phase III clinical studies to assess efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in patients with and without skin of color (SOC). The study was published in Dermatologic Surgery (April 2021)
For the post hoc analysis, Susan Taylor, MD, Pearl E. Grimes, MD, John H. Joseph, MD, Anneke Jonker, MSc, and Rui Avelar, MD, grouped patients by Fitzpatrick skin Type: IV + V + VI (with SOC) versus I + II + III (without SOC). The primary efficacy end point was the proportion of responders with a ≥1-point improvement from baseline at maximum frown on the 4-point Glabellar Line Scale (GLS). Adverse events (AEs) were also summarized.
All patients had received a single dose of 20U prabotulinumtoxinA, which was well tolerated and similar in effectiveness for the treatment of glabellar lines for patients with and without SOC, though there were some minor—but not statistically significant—differences in response rates and adverse events.
Responder rates among patients with SOC (n = 140) were lower than those without SOC (n = 352), by 5.9% on average across all visits; at no time point were differences statistically significant.
By day two, approximately half of all patients had achieved a ≥1-point improvement on the GLS at maximum frown by investigator assessment (47.0% with SOC and 52.8% without SOC). By day 30, 94.0% and 96.0% of those with and without SOC, respectively, had achieved the primary end point. At the end of study on Day 150, more than 30% of patients continued to show a ≥1-point improvement on the GLS at maximum frown: 31.5% with SOC and 40.1% without SOC.
The incidences of treatment-related AEs were similar among those with and without SOC: 14.3% versus 11.9%, respectively, with headache being the most common treatment-related AE, occurring in 12.1% of patient with SOC and 8.2% without SOC. Other AEs of particular interest—including eyelid ptosis (1.4%), brow ptosis (0.6%), blurred vision (0.6%) and diplopia (0.3%)—were uncommon and experienced only by patients without SOC.
The authors postulated “that these small but consistent differences in response between the two groups are explained, at least in part, by differences in the degree of previous botulinum toxin exposure recorded at baseline. In our study, compared with 30.0% of patients with SOC, 43.2% of patients without SOC reported previous exposure.”