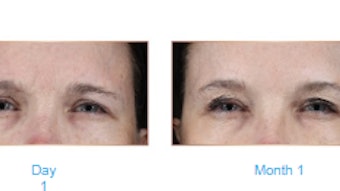
A comprehensive literature review, published in the April 2022 issue of Plastic and Reconstructive Surgery found that neutralizing antibody prevalence was “nil to insignificant” in hyperhidrosis and aesthetic indications, although significant in dystonia, spasticity and urologic conditions.
Related: Navigating Indications for Off-label Botulinum Toxin Use
Researchers from the U.K., India and Pakistan, performed five systematic reviews consisting of 203 studies (17,815 patients). They used the AMSTAR-2 (revised version of A Measurement Tool to Assess Systematic Reviews) tool for the critical appraisal of the selected systematic reviews.
They found that across the clinical indications, neutralizing antibody prevalence was significantly higher in dystonia, spasticity and urologic conditions, and nil to insignificant in hyperhidrosis and aesthetic indications. The overall rate for the neutralizing antibody formation across three different formulations, abobotulinumtoxinA, incobotulinumtoxinA and onabotulinumtoxinA, was 1% to 2.1%, with no significant difference between them. And the overall frequency of the development of neutralizing antibodies and the immunogenicity of abobotulinumtoxinA, incobotulinumtoxinA and onabotulinumtoxinA remain low to insignificant.
Related: [Botulinum Toxins] Tips To Reduce the Risk of Eyelid Ptosis
The authors encouraged properly designed comparative trials to further explore the difference in the prevalence of neutralizing antibodies across the commercially available botulinum toxin type A products as well as the relevance of neutralizing antibody titer to clinical responsiveness and nonresponse.