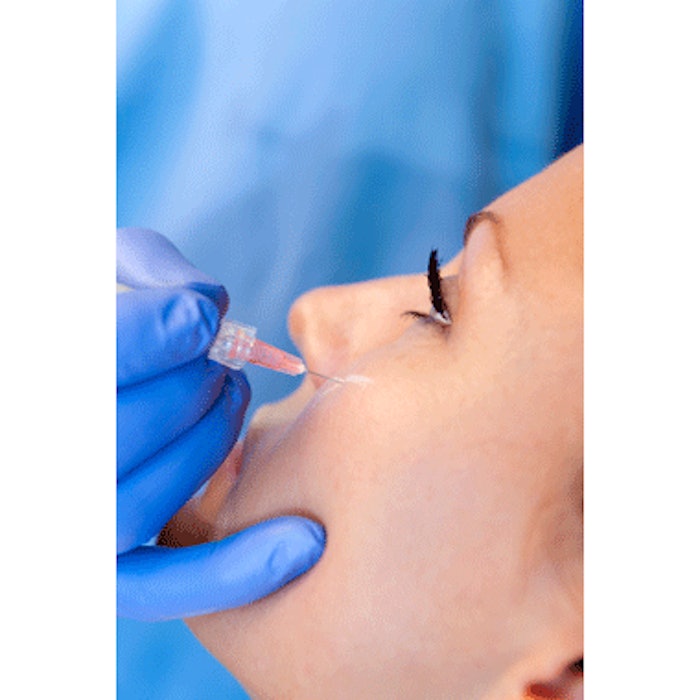
The U.S. Food and Drug Administration General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee has voted unanimously that the benefits of JUVEDERM VOLUMA XC—an injectable hyaluronic acid volume filler—outweigh the risks. The committee's recommendation, although not binding, will be considered by the FDA when making the final approval decision for JUVEDERM VOLUMA XC for cheek augmentation to correct age-related volume deficit in the mid-face. If approved by the FDA, Allergan anticipates launching the new filler in late 2013.
“Today's recommendation is an important step in the FDA review process for JUVEDERM VOLUMA XC," said Scott M. Whitcup MD, executive vice president of research and development and chief scientific officer, Allergan.











