Jeune Aesthetics, a subsidiary of Krystal Biotech, has announced positive proof-of-concept efficacy data from Cohort 2 of the PEARL-1 study of KB301, Jeune Aesthetics’ lead candidate for the treatment of aesthetic skin conditions.
KB301 is an injectable treatment designed to address declining levels of collagen by delivering the human COL3A1 gene to increase production of normal type III collagen at the site of administration. KB301 leverages Krystal’s proprietary gene delivery platform to restore protein production and rebuild the underlying extracellular matrix structure.
Related: Jeune Aesthetics Initiates Dosing in Collagen-stimulating KB301 Trial
About the PEARL-1 Trial
The Phase 1 dose-ranging trial evaluated the safety, tolerability and initial efficacy of intradermal injections of KB301 in adult subjects aged 18 to 75.
In Cohort 1, three different dose levels of KB301 were evaluated in seven healthy subjects who received two intradermal injections into healthy buttock tissue spaced 30 days apart. KB301 injected areas were compared to uninjected or saline injected control tissue within the same subject. Treatment and control sites were biopsied at day 2 or day 32. KB301 was shown to be well tolerated for COL3A1 supplementation in healthy human subjects.
Cohort 2 is a randomized, double-blind, placebo-controlled clinical trial that evaluated the safety and efficacy of KB301 for the improvement of fine lines and skin texture in the lower and upper cheek and for improvement in skin thickness in the knee.
Related: Krystal Biotech Launches Aesthetics-focused Subsidiary
Cohort 2 enrolled 27 subjects across two trial sites. Bilateral treatment areas included the neck behind the ear to assess initial safety and on the cheek below and above the zygomatic arch (lower and upper cheek), and around the knee. Subjects were randomized 2:1 to receive low dose KB301 or placebo in the upper cheek and knee as multiple microdepot injections using a 33 G needle.
Subjects receiving KB301 in the lower check were randomized 2:1 to receive either low dose KB301, high dose KB301 or placebo. Four patients dropped out of the Cohort 2 study—one subject following the initial safety assessment behind the ear, two subjects for unspecified reasons and one subject due to unevenness in the face between active and placebo administration sites during the study.
Above the Knee Safety and Efficacy Results
Low dose KB301 was well tolerated by subjects. (Subjects were not administered high dose of KB301 above the knee.) Adverse events included injection site reactions (ISRs) with 100% of the adverse events categorized as mild. The adverse events were transitory and dramatically reduced during follow-on injections.
Efficacy at Visit 6 was clinically meaningful across Subject Satisfaction Scores, Blinded Independent Reviewer Assessment and Mean Change in Skin Thickness:
Subject satisfaction scores showed a 21.9% responder rate difference between KB301 and placebo (41.9% for KB301 and 20% for placebo). Blinded independent reviewer assessment showed a 21.5% response rate difference (54.8% for KB301 and 33.3% for placebo). The mean change in skin thickness was 1.07mm between KB301 and placebo (KB301: 1.74mm, placebo: 0.67mm).
Related: Poly-l-lactic Acid Safe and Effective for Treating Upper Knee Skin Laxity
Lower Cheek Results
Both the high and the low dose of KB301 were well tolerated by subjects. Adverse events included injection site reactions with 91% of the adverse events categorized as mild and 9% moderate. The adverse events were transitory and dramatically reduced during follow-on injections.
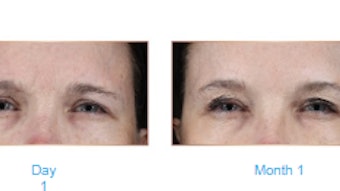
Efficacy at Visit 6 was clinically meaningful across subject satisfaction scores. Before and after picture evaluations showed clear improvement in both fine lines and skin texture in patients administered with high dose KB301.
Blinded independent reviewer assessments using Jeune’s Skin Roughness Score (JASRS) and Fine Lines Score (JAFLS) did not show clinical separation between active and placebo. These scales, developed specifically for this skin area, will be adapted specifically for KB301 by Jeune Aesthetics prior to advancing development.
Upper Cheek Results
Low dose KB301 was well tolerated by subjects. (Subjects were not administered high dose of KB301 in the upper cheek.) Adverse events included injection site reactions with 98% of the adverse events categorized as mild and 2% moderate. The adverse events were transitory and dramatically reduced during follow-on injections.
Efficacy at visit 6 was clinically meaningful across subject satisfaction scores. Before and after picture evaluations showed clear improvement in both fine lines and texture in patients administered with low dose of KB301. Blinded independent.t reviewer assessments using JASRS and JAFLS did not show clinical separation between active and placebo.
Related: Stromal Vascular Fraction Gel Injections Reverse Photoaging in Skin
“We are pleased to see results supporting the clinical benefits afforded by KB301, especially improvement of fine lines and texture in the cheek and improved thickness results in the knee, with only minimal adverse events across all injection sites,” said Bhushan Hardas, MD, president of Jeune Aesthetics. “We look forward to advancing KB301 into Phase 2 testing later this year, as well as progressing the rest of the Jeune Aesthetics’ pipeline as we work to create a new category of aesthetic medicine designed to address—and potentially reverse—biological changes in aging skin.”
Subjects from the PEARL-1 Cohort 2 trial will be enrolled in a durability trial to look for duration of effect, reduction of the unevenness in placebo-treated sites and for long term safety monitoring. Based on the results from Cohort 2, Jeune Aesthetics is currently planning two Phase 2a trials—one to improve skin quality attributes in the lower cheek and a second to evaluate KB301 for aesthetic treatment of the hands.