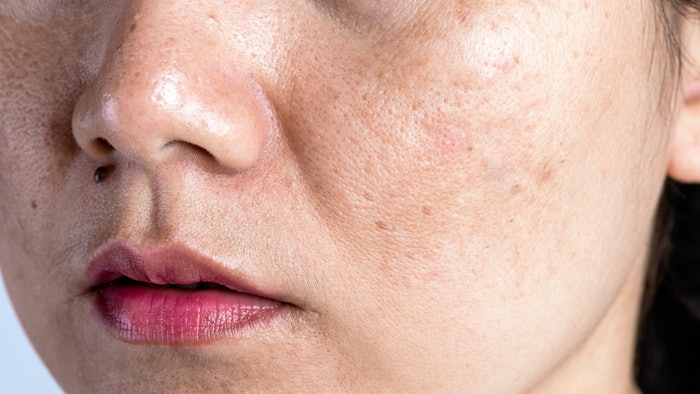
Low-fluence 1064 nm Q-switched Nd: YAG laser (LQSNY) can safely be used as an alternative treatment for Melasma and could have improved efficacy when combined with other lasers, according to a May 2022 study by Jiaoquan Chen and Nanji Yu, et. al. for the Journal of Cosmetic Dermatology.
Although the safety was confirmed, the efficacy of LQSNY monotherapy showed no statistically significant improvement compared to other lasers or drugs. The combined therapy showed an improved melasma area and severity index (MASI), but no substantial improvement in melanin index and self-assessment.
Related: Cysteamine vs. Hydroquinone/Ascorbic Acid for Epidermal Melasma
The project included 12 studies comprising 358 patients. No significant differences in MASI were observed between the LQSNY and drug groups (mean difference (MD):−0.26, 95% confidence interval (CI):−1.16–0.64, p = 0.57), and melasma improvement (MI) showed little difference between LQSNY and LQSNY combined with other lasers (MD 0.05, 95% CI:−0.61, 0.70, p = 0.56).
Combination therapy with LQSNY and drugs had a greater MASI improvement compared with LQSNY therapy alone (MD: 1.78, 95% CI 0.93–2.63, p < 0.0001) but showed no statistically significant results in melanin index (MI) and self-assessment. LQSNY with intense pulsed light provided an added benefit for melasma severity (MD:3.23, 95% CI:0.65–5.81, p = 0.01).
The results indicate that a low-fluence 1064 nm Q-switched Nd: YAG laser can be applied as an alternative treatment for drug intolerance. Combination therapy with LQSNY and drugs or other lasers may have improved efficacy, but more data is required to verify the claim.