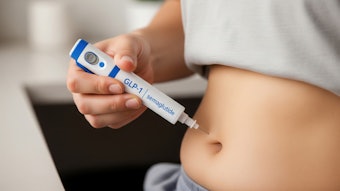
Allergan Aesthetics announced the U.S. FDA approval of SKINVIVE by JUVÉDERM to improve skin smoothness of the cheeks in adults over the age of 21. SKINVIVE by JUVÉDERM is the first and only hyaluronic acid (HA) intradermal microdroplet injection for skin smoothness available in the U.S. with results lasting through six months with optimal treatment.
SKINVIVE by JUVÉDERM is a smooth, injectable HA gel that contains a small amount of local anesthetic (lidocaine). Unlike other facial injectables that enhance and augment the treatment area, SKINVIVE by JUVÉDERM improves skin quality in the cheeks by smoothing the skin and increasing hydration.
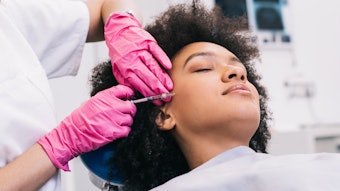
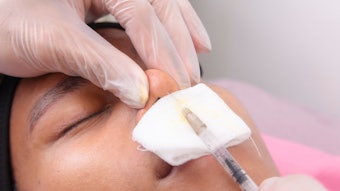
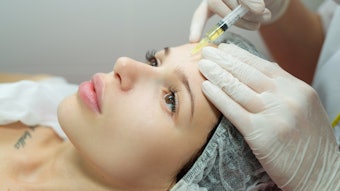
SKINVIVE by JUVÉDERM is a specialized, smooth, hydrating gel that is absorbed easily into the skin and is approved for all Fitzpatrick Skin Types I-VI, lightest to darkest, addressing an important unmet need in the skin quality category. The product was designed with global skin health experts to improve smoothness of the cheeks leading to a lasting glow. Photo courtesy of SKINVIVE by JUVÉDERM
Photo courtesy of SKINVIVE by JUVÉDERM
"Skin quality is among the top concerns my patients express when seeking aesthetic treatments. It's an extremely important factor I consider in my therapeutic process of restoring their natural beauty and appearance," said Macrene Alexiades, M.D., Ph.D., dual U.S.-EU board-certified dermatologist, SKINVIVE by JUVÉDERM lead investigator, and author of the clinical trial published in Dermatologic Surgery. "One key way to improve skin quality is by enhancing hydration. SKINVIVE by JUVÉDERM is truly innovative because it works beneath the skin's surface to increase skin hydration improving skin quality. I am excited to add this unique offering to my dermatologic treatment regimen for my patients."
Related: Two Molluscum Contagiosum NDAs Approved by FDA
In a European post-marketing study, changes in aquaporin were observed after SKINVIVE by JUVÉDERM treatment. Allergan Aesthetics is looking to pursue further research to better understand the significance of the changes in aquaporin observed after SKINVIVE by JUVÉDERM treatment.
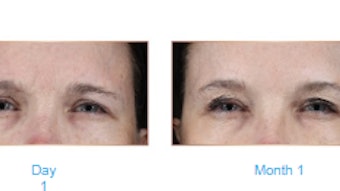
In a randomized, multicenter, evaluator-blinded, controlled pivotal clinical study, 58% and 56% of patients treated with SKINVIVE by JUVÉDERM saw a ≥ 1 point improvement on the Allergan Cheek Smoothness Scale (ACSS) at one month and six months respectively. In a patient reported satisfaction with skin questionnaire, 63% of patients were satisfied with how radiant their facial skin looked at six months compared with 11% before treatment.
At six months, 72% were satisfied with how hydrated their facial skin looked at six months compared to 24% before treatment. Additionally, 69% were satisfied with how refreshed their facial skin made them look at six months compared to 16% before treatment. At month six, 83% were satisfied with how healthy their facial skin looked compared to 38% before treatment.