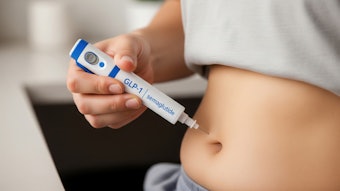
As semaglutide continues to take over the medical aesthetics industry, its use is potentially expanding beyond just diabetes and weight loss. Clinical trials are underway testing GLP-1 drugs to treat Alzheimer's, Parkinson's and even drug addiction, according to Lexaria, which offers a GLP-1 drug that uses a DehydraTECH drug-delivery formulation and processing technology to improve both delivery and efficacy. The company's study on the drug suggested potential indications of therapeutic efficacy in heart disease and chronic kidney disease, as well as a wide range of other conditions.
Ozempic is close to mainstream status and has reached beyond the niche audience, extending its reach into the fitness industry. The catalyst for its injection into the U.S. fitness industry was when Life Time Fitness announced plans for a program to prescribe weight loss injections for members through in-house medical teams, according to Life Time president and COO Jeff Zwiefel.
Related: The Potential Long-term Industry Effects of the LifeTime Fitness Ozempic Pilot Program
Lexaria's studies go beyond weight loss to investiage the efficacy of GLP-1 drugs on kidney disease, liver disease, bone health and even aging. The company's initial focus is on diabetes and obesity. The company just received the final results from its first human pilot study of GLP-1 and indicated the results were positive.
The study was performed by a university research center that compared a control group receiving a single dose of a Rybelsus tablet to a treatment group receiving a matching dose of Rybelsus that had been formulated using DehydraTECH processing-technology enhancements. Per the study, 24 hours after administration, blood glucose levels in the Rybelsus control group were unchanged from baseline, compared to a 5.01% reduction for the DehydraTECH GLP-1 treatment group. The data showed the control group experienced a 21.7% spike in glucose levels after a meal, versus just a 6.2% increase in the DehydraTECH treatment group.
GLP-1 Market Growth
Lexaria's results showed that DehydraTECH-processed Rybelsus oral semaglutide was more effective at controlling blood glucose than standard Rybelsus alone. Rybelsus is one of the most popular drugs in the diabetes market, posting sales of $1.63 billion in 2022 and already posting $1.234 billion in sales during just the first half of 2023, according to the study. By comparison, Ozempic posted sales of $6.174 billion in the first half of 2023.
According to a report from Research And Markets, the global Glucagon-like Peptide 1 (GLP-1) market is set to attain a market value of US$18.75 billion by 2023, driven by a robust Compound Annual Growth Rate (CAGR) of 6.48% during the projected period.
Lexaria is planning to begin more human pilot studies expected to test at least one additional GLP-1 drug as well as test an oral dissolvable that is not required to be swallowed. In Q2 2024, Lexaria intends to study DehydraTECH-GLP-1 in a multiweek human clinical trial to examine both diabetes-related control (in part via reduced blood sugar levels) as well as weight loss and side effects.
Safety Issues with GLP-1 Drugs
While GLP-1 drugs are touted for their efficacy and compelling safety profile, they still can trigger nausea, vomiting, diarrhea and more. In addition, ongoing research is examining long-term effects related to bone density and muscle loss. Improving side effects is critical for market share as the GLP-1 market matures. Consumers are even gravitating toward cheaper counterfeit versions of Ozempic and off-brand medications, which has spurred lawsuits from Novo Nordisk.
In a consumer survey conducted by 9am Health, 38% of respondents have tried knock-off GLP-1s or black market weight loss products that have not gone through FDA approval in the past.
"As more weight loss management medications receive FDA approval, our data shows that people are increasingly eager to get their hands on these drugs for weight loss, even if it means making extreme sacrifices like cutting back on entertainment and travel or changing jobs," says Avantika Waring, M.D., chief medical officer at 9amHealth. "As demand continues through 2024, 9amHealth is continuously working to help employers keep up by offering their employees effective alternatives to weight loss drugs all in one place and at a lower cost."
Eli Lilly Comes Out Against Cosmetic Use of GLP-1
The issue with GLP-1 pricing and lack of insurance coverage presents a potential issue for the drug's national growth. According to FDA news Eli Lilly's LillyDirect will allow patients to order 14 medications for home delivery, twelve of which are various types of insulin, all capped at $35 per month. The program also includes the weight loss drug Zepbound (tirzepatide) and one migraine medication, Emgality (galcenezumab).
Eli Lilly recently launched a public service campaign against using the GLP-1 inhibitor and other Lilly weight-loss drugs simply for cosmetic purposes.
The knockoff medications are more dangerous as well. According to data from America's Poison Centers, there were nearly 3,000 calls involving semaglutide from January through November 2023, a 15x increase since 2019. In 94% of calls, semaglutide was the only substance reported.
These calls are mostly from adults ages 40 to 70, with the largest group in the 60-to-69 range, per CNN. They also aren’t only related to injections of compounded forms of the drug, but also to the click pen that comes with the prescription drug. In one case, a caller said they were having trouble with the pen and accidentally gave themselves an entire month of doses at once.
As GLP-1 drugs continue to grow in popularity and impact society, there is a clear need for stronger regulations and increased public awareness of exactly what semaglutide is and how it can affect the body.