NanoPass Technologies Ltd. plans to officially launch MicronJet 800 during IMCAS World 2026 on Jan. 29 through Jan. 31 in Paris.
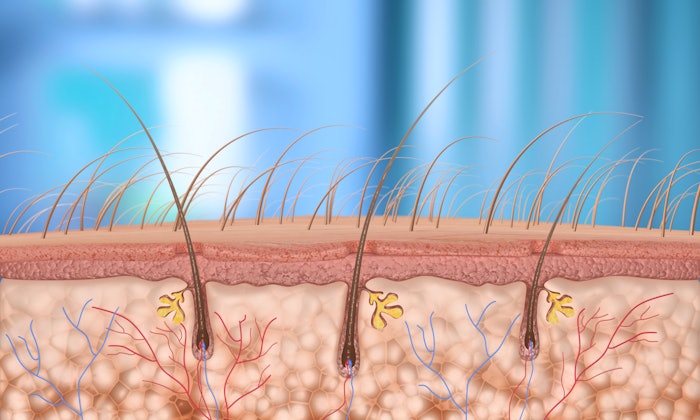
Intended for non-surgical hair restoration, the new microneedle-based delivery technology was designed for areas with thicker skin. However, the Micronjet 800 is suitable for additional aesthetic and dermatological applications, including jawline and cheek treatments, hyperhidrosis and scar revision, according to a Jan. 14 press release.
 NanoPass Technologies introduces the MicronJet 800, a next-generation microneedle device for precise, nearly painless intradermal delivery.Courtesy of NanoPass Technologies Ltd. Jan. 14 press release
NanoPass Technologies introduces the MicronJet 800, a next-generation microneedle device for precise, nearly painless intradermal delivery.Courtesy of NanoPass Technologies Ltd. Jan. 14 press release
The MicronJet 800 and its 0.8 mm intradermal delivery, on the other hand, can be used for medium-to-high viscosity formulations, providing a flow rate comparable to a 27-gauge needle while ensuring precise intradermal placement. The device offers consistent injections and is compatible with standard Luer-lock or Luer-slip syringes.
Like the 600, the new device uses silicon-crystal delivery pyramids produced with semiconductor-grade MEMS technology to resist blunting. Undergoing 70 clinical studies involved in vaccines, therapeutics, like allergy testing, and aesthetic applications, the device is compatible with non-cross-linked HA, skin boosters, polynucleotides, microtoxin, mesotherapy cocktails, PRP, iPRF, corticosteroids and lidocaine.
“MicronJet ensures effective intradermal placement while maintaining comfort, making it an ideal solution for hair restoration treatments," said Alberto Diaspro, MD, a Maxillofacial Plastic Surgeon from Italy.